Harnessing the Immune System: Innovations in Cancer Immunotherapy
The Role of Immune Checkpoint Inhibitors, CAR T-Cell Therapy, Cancer Vaccines, and More
Introduction
Immunotherapy represents one of the most promising advancements in cancer treatment, leveraging the body's immune system to identify and eradicate cancer cells. This article explores the various types of immunotherapies, including immune checkpoint inhibitors, CAR T-cell therapy, cancer vaccines, cytokine therapy, and oncolytic virus therapy. Each of these therapies has unique mechanisms and potential benefits, contributing to the evolving landscape of oncology.
Immune Checkpoint Inhibitors
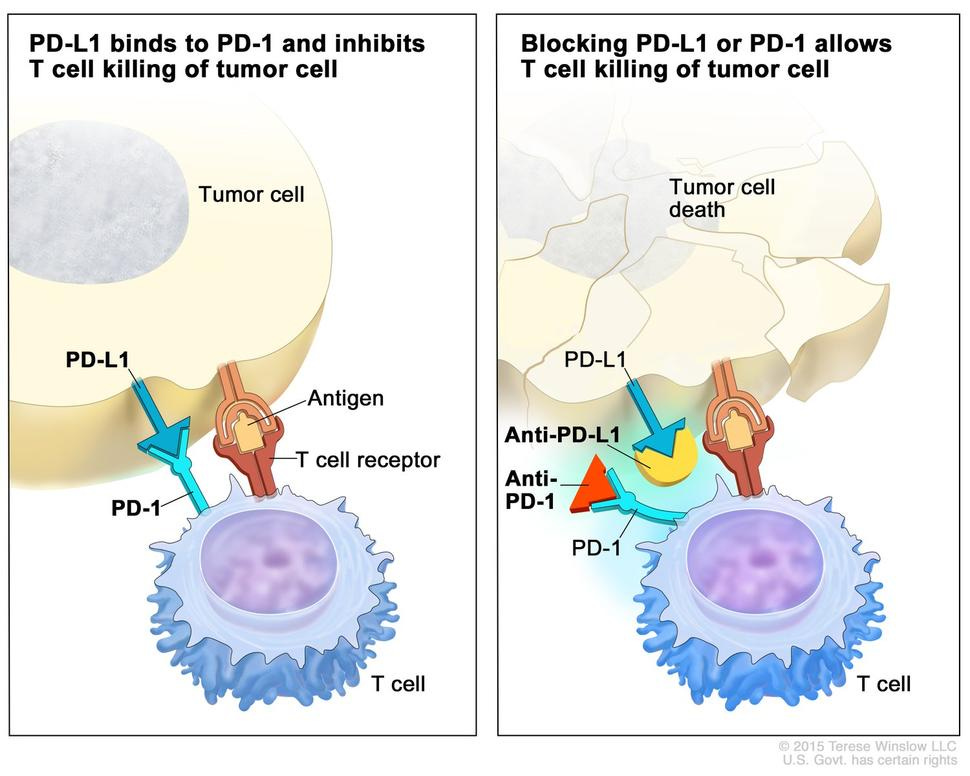
Immune checkpoint inhibitors have revolutionized the treatment of various cancers by blocking proteins that prevent the immune system from attacking cancer cells. These inhibitors target molecules such as PD-1, PD-L1, and CTLA-4, enhancing the immune response against tumors. Drugs like nivolumab and pembrolizumab have shown remarkable efficacy in treating melanoma, non-small cell lung cancer, and renal cell carcinoma.1-3 These inhibitors work by disrupting the "off" signals that cancer cells send to evade immune detection, thereby allowing the immune system to recognize and destroy cancer cells.4
Despite their success, some patients do not respond to checkpoint inhibitors, and researchers are investigating combination therapies to overcome resistance. Combining checkpoint inhibitors with other treatments, such as targeted therapies, radiation, or additional immunotherapies, has shown promise in preclinical and clinical studies.5-7 For example, combining PD-1 inhibitors with CTLA-4 inhibitors has improved outcomes in melanoma patients.8
Additionally, researchers are exploring the mechanisms of resistance to checkpoint inhibitors. Some tumors develop resistance through genetic mutations or alterations in the tumor microenvironment that suppress immune responses. Understanding these mechanisms is crucial for developing new strategies to overcome resistance and enhance the effectiveness of checkpoint inhibitors. Ongoing studies are investigating biomarkers that predict response to checkpoint inhibitors, which could help tailor treatments to individual patients and improve outcomes.9
CAR T-Cell Therapy
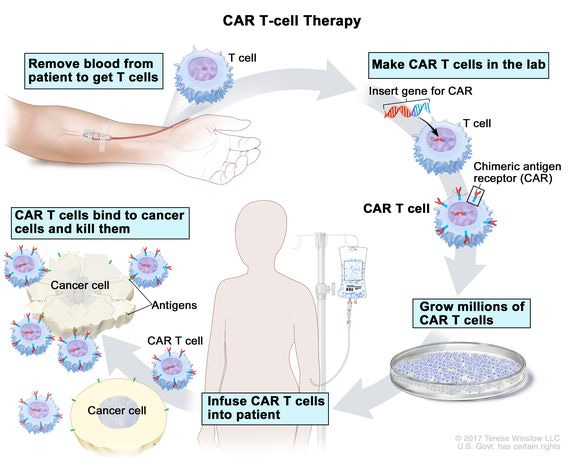
Chimeric Antigen Receptor (CAR) T-cell therapy involves engineering a patient’s T cells to express a receptor specific to cancer cells, thereby enhancing the immune system's ability to target and destroy these cells.10,11 CAR T-cell therapy has been particularly successful in treating certain hematologic cancers, such as acute lymphoblastic leukemia and non-Hodgkin lymphoma.12,13
The process involves extracting T cells from the patient, genetically modifying them to express the CAR, and reinfusing them into the patient. These modified T cells then seek out and destroy cancer cells.14 While CAR T-cell therapy has shown remarkable efficacy, it can cause severe side effects, including cytokine release syndrome (CRS) and neurotoxicity.15,16 Researchers are working on improving the safety and expanding the applicability of CAR T-cell therapy to solid tumors.17,18
To enhance the efficacy of CAR T-cell therapy for solid tumors, scientists are developing new strategies, such as dual CAR T cells that target multiple antigens on tumor cells, or using gene editing technologies like CRISPR to improve T cell persistence and function. Additionally, combining CAR T-cell therapy with other treatments, such as checkpoint inhibitors or oncolytic viruses, is being explored to enhance the anti-tumor immune response and overcome the immunosuppressive tumor microenvironment.19
Cancer Vaccines
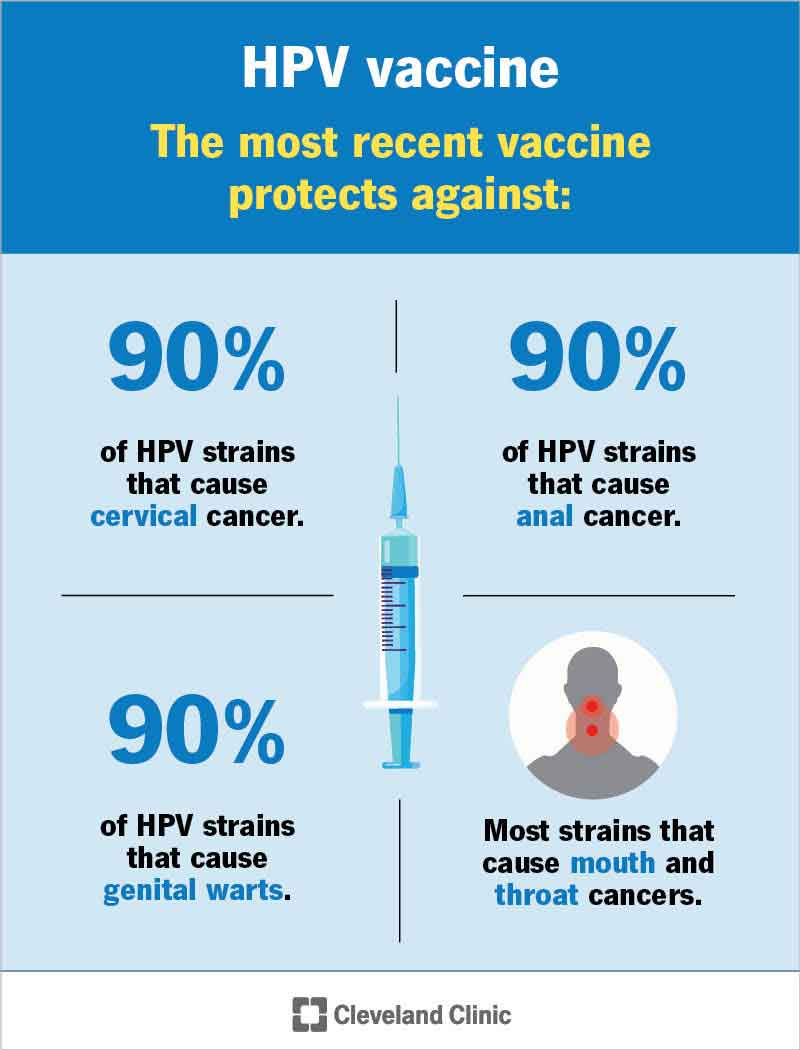
Cancer vaccines are designed to stimulate the immune system to attack cancer cells by presenting them with specific antigens associated with tumors.20,21 These vaccines can be prophylactic, aiming to prevent cancer development (such as the HPV vaccine), or therapeutic, aiming to treat existing cancers.22 Therapeutic cancer vaccines have shown promise in various cancers, including prostate cancer and melanoma.23,24
Recent advances in mRNA technology, exemplified by the COVID-19 vaccines, have accelerated the development of mRNA-based cancer vaccines. These vaccines can be rapidly designed and produced, offering a flexible platform for targeting a wide range of tumor antigens.25,26 Clinical trials are ongoing to evaluate the efficacy of these novel vaccine strategies.27,28
Furthermore, personalized cancer vaccines are being developed using neoantigens, which are unique mutations found in an individual’s tumor. These personalized vaccines are tailored to the specific genetic profile of the patient’s cancer, potentially enhancing the immune response and improving treatment outcomes.29 Combining cancer vaccines with other immunotherapies, such as checkpoint inhibitors, is also being investigated to boost the immune response and achieve more durable anti-tumor effects.30
Cytokine Therapy
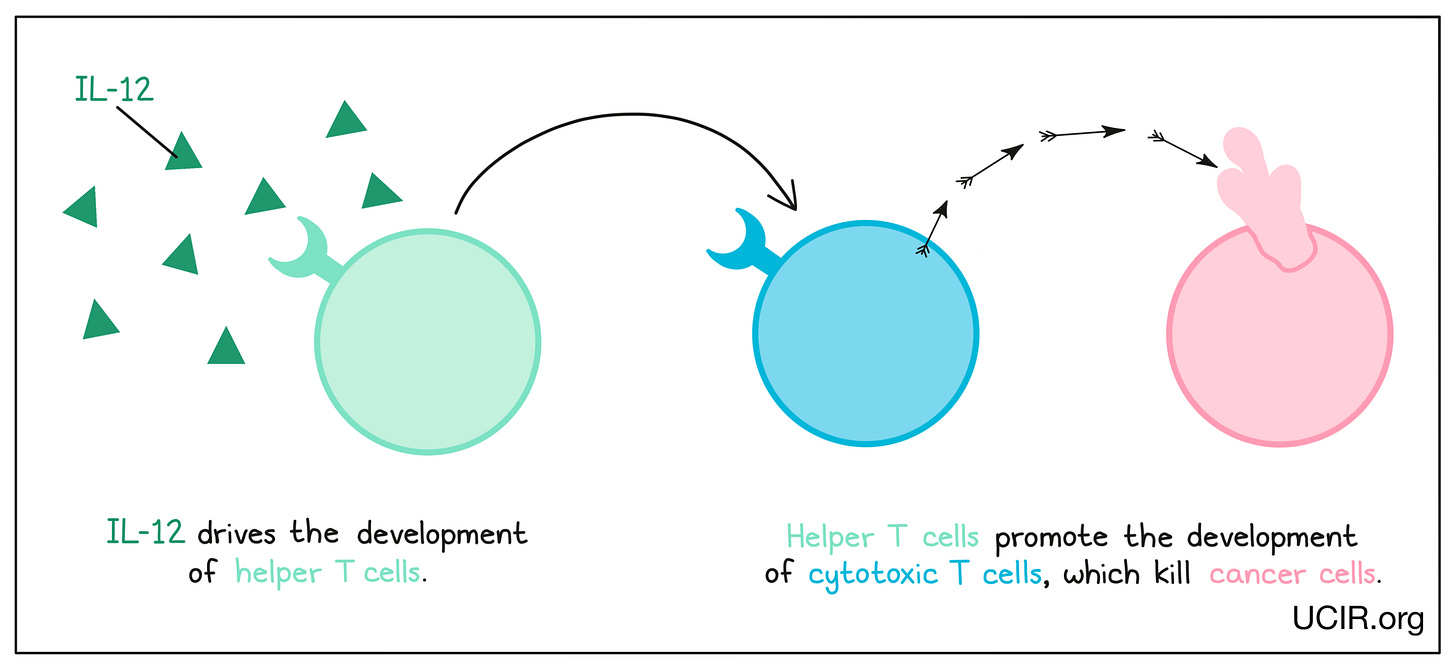
Cytokines are signaling proteins that modulate the immune system's activity. In cancer therapy, cytokines such as interleukins and interferons are used to enhance the immune response against tumors.31,32 Interleukin-2 (IL-2) and interferon-alpha (IFN-α) were among the first cytokines used in cancer immunotherapy and have been approved for treating cancers such as melanoma and renal cell carcinoma.33
However, cytokine therapy can cause severe systemic side effects due to its broad activation of the immune system. To address this, researchers are developing targeted cytokine therapies and engineering cytokines with reduced toxicity.34,35 Recent advancements include the development of cytokine-anchored nanoparticles and fusion proteins that localize the cytokine activity to the tumor microenvironment.36,37
Additionally, new cytokines with enhanced therapeutic properties are being designed using protein engineering techniques. These engineered cytokines can selectively activate immune cells within the tumor microenvironment while minimizing systemic toxicity.38 Combining cytokine therapy with other immunotherapies, such as checkpoint inhibitors or adoptive cell transfer, is also being explored to enhance anti-tumor responses and overcome immune resistance.39
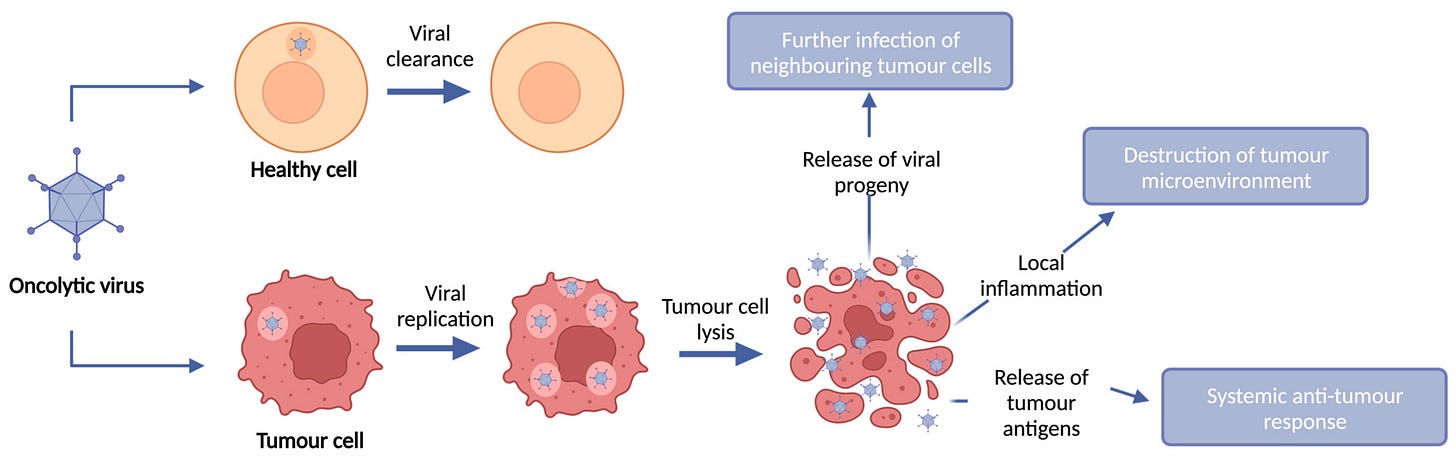
Oncolytic Virus Therapy
Oncolytic viruses are genetically modified viruses that selectively infect and kill cancer cells while sparing normal cells.40,41 These viruses can also stimulate an immune response against the tumor by releasing tumor antigens as the infected cancer cells are destroyed.41 One example is Talimogene laherparepvec (T-VEC) is an FDA-approved oncolytic virus therapy for treating melanoma.42
Researchers are exploring combinations of oncolytic viruses with other immunotherapies, such as checkpoint inhibitors, to enhance their efficacy.43,44 Additionally, advances in genetic engineering are allowing for the creation of more potent oncolytic viruses with enhanced tumor-targeting capabilities.45
Challenges and Future Directions
Despite the successes of immunotherapy, several challenges remain. These include understanding and overcoming resistance mechanisms, managing side effects, and improving the efficacy of immunotherapies for solid tumors. Additionally, the high cost of immunotherapy treatments poses a significant barrier to widespread adoption.
Ongoing research aims to address these challenges by developing combination therapies, personalized treatment approaches, and novel immunotherapy modalities. Advances in biomarker discovery and precision medicine are expected to play a crucial role in tailoring immunotherapy to individual patients, thereby maximizing efficacy and minimizing adverse effects.
Conclusion
Immunotherapy has transformed the field of oncology, offering new hope for patients with various types of cancer. The diverse strategies discussed in this article—immune checkpoint inhibitors, CAR T-cell therapy, cancer vaccines, cytokine therapy, and oncolytic virus therapy—highlight the rapid advancements and the potential for immunotherapy to become a cornerstone of cancer treatment. As research continues, these therapies will likely become more effective, safer, and accessible, bringing us closer to the ultimate goal of curing cancer.
References
Huang, A.C., Zappasodi, R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol 23, 660–670 (2022). https://doi.org/10.1038/s41590-022-01141-1
Immune checkpoint inhibitors. NCI. Accessed July 7, 2024. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/checkpoint-inhibitors.
June 5 2024, May 3 2024, May 1 2024. Long-term side effects of immune checkpoint inhibitors. Long-Term Side Effects of Immune Checkpoint Inhibitors - NCI. Accessed July 7, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2021/immune-checkpoint-inhibitors-melanoma-long-term-side-effects.
Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun 11, 3801 (2020). https://doi.org/10.1038/s41467-020-17670-y
Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299-308. doi:10.1038/nrc2355
Zhang, Z., Liu, X., Chen, D. et al. Radiotherapy combined with immunotherapy: the dawn of cancer treatment. Sig Transduct Target Ther 7, 258 (2022). https://doi.org/10.1038/s41392-022-01102-y
Klein-Brill, A., Amar-Farkash, S., Rosenberg-Katz, K. et al. Comparative efficacy of combined CTLA-4 and PD-1 blockade vs. PD-1 monotherapy in metastatic melanoma: a real-world study. BJC Rep 2, 14 (2024). https://doi.org/10.1038/s44276-024-00041-1
Propper, D.J., Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat Rev Clin Oncol 19, 237–253 (2022). https://doi.org/10.1038/s41571-021-00588-9
Car T cells: Engineering immune cells to treat cancer. CAR T Cells: Engineering Immune Cells to Treat Cancer - NCI. Accessed July 7, 2024. https://www.cancer.gov/about-cancer/treatment/research/car-t-cells.
Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13:925985. Published 2022 Jul 22. doi:10.3389/fimmu.2022.925985
Jogalekar MP, Rajendran RL, Khan F, Dmello C, Gangadaran P, Ahn BC. CAR T-Cell-Based gene therapy for cancers: new perspectives, challenges, and clinical developments. Front Immunol. 2022;13:925985. Published 2022 Jul 22. doi:10.3389/fimmu.2022.925985
Sterner, R.C., Sterner, R.M. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 11, 69 (2021). https://doi.org/10.1038/s41408-021-00459-7
Tian, Y., Xie, D. & Yang, L. Engineering strategies to enhance oncolytic viruses in cancer immunotherapy. Sig Transduct Target Ther 7, 117 (2022). https://doi.org/10.1038/s41392-022-00951-x
June 5 2024, May 3 2024, May 1 2024. Oncolytic virus disrupts immune-blocking protein. Oncolytic Virus Disrupts Immune-Blocking Protein - NCI. Accessed July 7, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2023/oncolytic-virus-blocking-tgf-beta.
Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine Release Syndrome: Current Perspectives. Immunotargets Ther. 2019;8:43-52. Published 2019 Oct 29. doi:10.2147/ITT.S202015
Zolaly MA, Mahallawi W, Khawaji ZY, Alahmadi MA. The Clinical Advances of Oncolytic Viruses in Cancer Immunotherapy. Cureus. 2023;15(6):e40742. Published 2023 Jun 21. doi:10.7759/cureus.40742
Fan, T., Zhang, M., Yang, J. et al. Therapeutic cancer vaccines: advancements, challenges, and prospects. Sig Transduct Target Ther 8, 450 (2023). https://doi.org/10.1038/s41392-023-01674-3
Drew L. Cancer-vaccine trials give reasons for optimism. Nature News. March 27, 2024. Accessed July 7, 2024. https://www.nature.com/articles/d41586-024-00840-z.
Li B, Jin J, Guo D, Tao Z, Hu X. Immune checkpoint inhibitors combined with targeted therapy: The recent advances and future potentials. Cancers. 2023;15(10):2858. doi:10.3390/cancers15102858
Marei, H.E., Hasan, A., Pozzoli, G. et al. Cancer immunotherapy with immune checkpoint inhibitors (ICIs): potential, mechanisms of resistance, and strategies for reinvigorating T cell responsiveness when resistance is acquired. Cancer Cell Int 23, 64 (2023). https://doi.org/10.1186/s12935-023-02902-0
June 5 2024, May 3 2024, May 1 2024. Is immunotherapy the only treatment some people need? Is Immunotherapy the Only Treatment Some People Need? - NCI. Accessed July 7, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2023/neoadjuvant-immunotherapy-only-treatment.
Morotti, M., Albukhari, A., Alsaadi, A. et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer 124, 1759–1776 (2021). https://doi.org/10.1038/s41416-021-01353-6
Majumder A. Evolving car-T-cell therapy for cancer treatment: From scientific discovery to cures. Cancers. 2023;16(1):39. doi:10.3390/cancers16010039
Waldmann TA. Cytokines in Cancer Immunotherapy. Cold Spring Harb Perspect Biol. 2018;10(12):a028472. Published 2018 Dec 3. doi:10.1101/cshperspect.a028472
Berraondo, P., Sanmamed, M.F., Ochoa, M.C. et al. Cytokines in clinical cancer immunotherapy. Br J Cancer 120, 6–15 (2019). https://doi.org/10.1038/s41416-018-0328-y
Conlon KC, Miljkovic MD, Waldmann TA. Cytokines in the Treatment of Cancer. J Interferon Cytokine Res. 2019;39(1):6-21. doi:10.1089/jir.2018.0019
Barbier, A.J., Jiang, A.Y., Zhang, P. et al. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol 40, 840–854 (2022). https://doi.org/10.1038/s41587-022-01294-2
Wang B, Pei J, Xu S, Liu J, Yu J. Recent advances in mRNA cancer vaccines: meeting challenges and embracing opportunities. Front Immunol. 2023;14:1246682. Published 2023 Sep 6. doi:10.3389/fimmu.2023.1246682
Immunotherapy for cancer. Immunotherapy for Cancer - NCI. Accessed July 7, 2024. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy.
June 5 2024, May 3 2024, May 1 2024. How mrna vaccines might help treat cancer. How mRNA Vaccines Might Help Treat Cancer - NCI. Accessed July 7, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2022/mrna-vaccines-to-treat-cancer.
Koury J, Lucero M, Cato C, et al. Immunotherapies: Exploiting the Immune System for Cancer Treatment. J Immunol Res. 2018;2018:9585614. Published 2018 Mar 14. doi:10.1155/2018/9585614
Aricò E, Castiello L, Capone I, Gabriele L, Belardelli F. Type I Interferons and Cancer: An Evolving Story Demanding Novel Clinical Applications. Cancers (Basel). 2019;11(12):1943. Published 2019 Dec 4. doi:10.3390/cancers11121943
June 5 2024, May 3 2024, May 1 2024. Timing and sequence critical for immunotherapy combination. NCI. Accessed July 7, 2024. https://www.cancer.gov/news-events/cancer-currents-blog/2017/combining-checkpoint-inhibitors.
Adu-Berchie, K., Brockman, J.M., Liu, Y. et al. Adoptive T cell transfer and host antigen-presenting cell recruitment with cryogel scaffolds promotes long-term protection against solid tumors. Nat Commun 14, 3546 (2023). https://doi.org/10.1038/s41467-023-39330-7
Deckers, J., Anbergen, T., Hokke, A.M. et al. Engineering cytokine therapeutics. Nat Rev Bioeng 1, 286–303 (2023). https://doi.org/10.1038/s44222-023-00030-y
Tojjari A, Saeed A, Singh M, Cavalcante L, Sahin IH, Saeed A. A Comprehensive Review on Cancer Vaccines and Vaccine Strategies in Hepatocellular Carcinoma. Vaccines (Basel). 2023;11(8):1357. Published 2023 Aug 12. doi:10.3390/vaccines11081357
Ling SP, Ming LC, Dhaliwal JS, et al. Role of immunotherapy in the treatment of cancer: A systematic review. Cancers. 2022;14(21):5205. doi:10.3390/cancers14215205
Ren Z, Zhang X, Fu Y-X. Facts and hopes on chimeric cytokine agents for cancer immunotherapy. Clinical Cancer Research. 2024;30(10):2025-2038. doi:10.1158/1078-0432.ccr-23-1160
Maeng HM, Berzofsky JA. Strategies for developing and optimizing cancer vaccines. F1000Res. 2019;8:F1000 Faculty Rev-654. Published 2019 May 13. doi:10.12688/f1000research.18693.1
Lin, D., Shen, Y. & Liang, T. Oncolytic virotherapy: basic principles, recent advances and future directions. Sig Transduct Target Ther 8, 156 (2023). https://doi.org/10.1038/s41392-023-01407-6
Shalhout, S.Z., Miller, D.M., Emerick, K.S. et al. Therapy with oncolytic viruses: progress and challenges. Nat Rev Clin Oncol 20, 160–177 (2023). https://doi.org/10.1038/s41571-022-00719-w
Hemminki, O., dos Santos, J.M. & Hemminki, A. Oncolytic viruses for cancer immunotherapy. J Hematol Oncol 13, 84 (2020). https://doi.org/10.1186/s13045-020-00922-1
Ren Y, Miao JM, Wang YY, et al. Oncolytic viruses combined with immune checkpoint therapy for colorectal cancer is a promising treatment option. Front Immunol. 2022;13:961796. Published 2022 Jul 15. doi:10.3389/fimmu.2022.961796
Zou H, Mou XZ, Zhu B. Combining of Oncolytic Virotherapy and Other Immunotherapeutic Approaches in Cancer: A Powerful Functionalization Tactic. Glob Chall. 2022;7(1):2200094. Published 2022 Oct 20. doi:10.1002/gch2.202200094
Muthukutty P, Yoo SY. Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective. Viruses. 2023;15(8):1645. Published 2023 Jul 28. doi:10.3390/v15081645